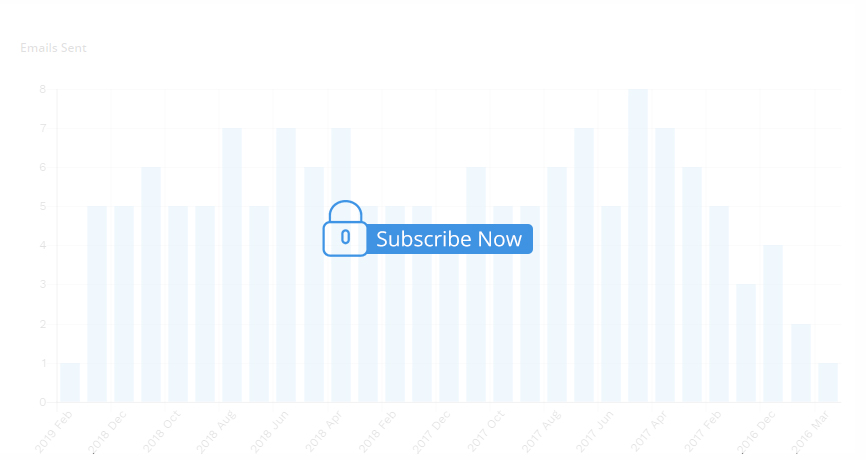
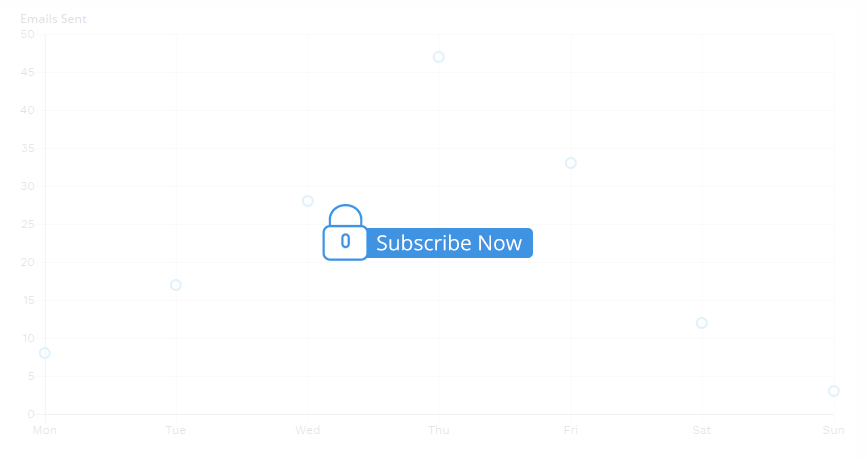
2024 Jun
Subject Analysis
Subjects Using Question
Average Subject Length
Subjects using emojis
Content Analysis
Subscribe Now
Average Reading Time
Subscribe Now
Average Word Length
Subscribe Now
Mobile Optimised Emails
Subscribe Now
Flesch Reading Score
Top Font Used
| No. | Font Name | |
Subscribe Now | ||
Top Keywords
| Keywords | Density |
Subscribe Now | |
We are not compliance4all.com and do not send emails on behalf of compliance4all.com.
If you wish to unsubscribe or stop receiving emails from compliance4all.com, please follow one of the steps below:
If you wish to unsubscribe or stop receiving emails from compliance4all.com, please follow one of the steps below:
- look for the 'unsubscribe' link in the email that you received. Click on the link and follow the steps to unsubscribe.
- sign in with your compliance4all.com account username into compliance4all.com and go to 'My Account' page. Look for the 'Email Preference' section or similar. Then follow the instruction to change your email preference or unsubscribe.